DVT Prophylaxis in Hospitalized Medical Patients - Questioning the Evidence
Early in my training I was taught that DVT prophylaxis was a crucial "bottom of the list" item. In the ensuing years, has my diligent adherence to this metric improved outcomes? I'm not sure.
Venous thromboembolism (VTE) chemoprophylaxis (aka “DVT prophylaxis”) for hospitalized medical patients is standard practice. Guidelines (ASH, ACCP, Thrombosis Canada) and common point-of-care resources (UpToDate) recommend its use, and the Joint Commission includes VTE prophylaxis among its Electronic Clinical Quality Measures (eCQMs) for Accreditation. And yet, when one examines the strongest evidence for VTE chemoprophylaxis, a curious pattern emerges. The landmark studies upon which this practice rests are largely driven by an end-point that we do not test for and which patients have no clear interest in our preventing. To understand how this occurred, we’ll need to review the history, evidence, and pathophysiology of this important condition.
Emergence of Prophylaxis in Medical Patients
Recognition that acutely ill, immobilized medical inpatients die from seemingly preventable pulmonary emboli can be traced back more than half a century. Autopsy-based observations and the landmark controlled anticoagulation trial by Barritt and Jordan in 1960 sounded the first alarm that VTE was a hospital hazard, even outside the operating theatre. For decades, however, preventive efforts remained almost entirely surgical, and it was not until the 1970s, when epidemiologic series showed similar deep venous thrombosis (DVT) rates in patients admitted for acute myocardial infarction and stroke, that systematic prophylaxis of medical cohorts began to attract serious study.
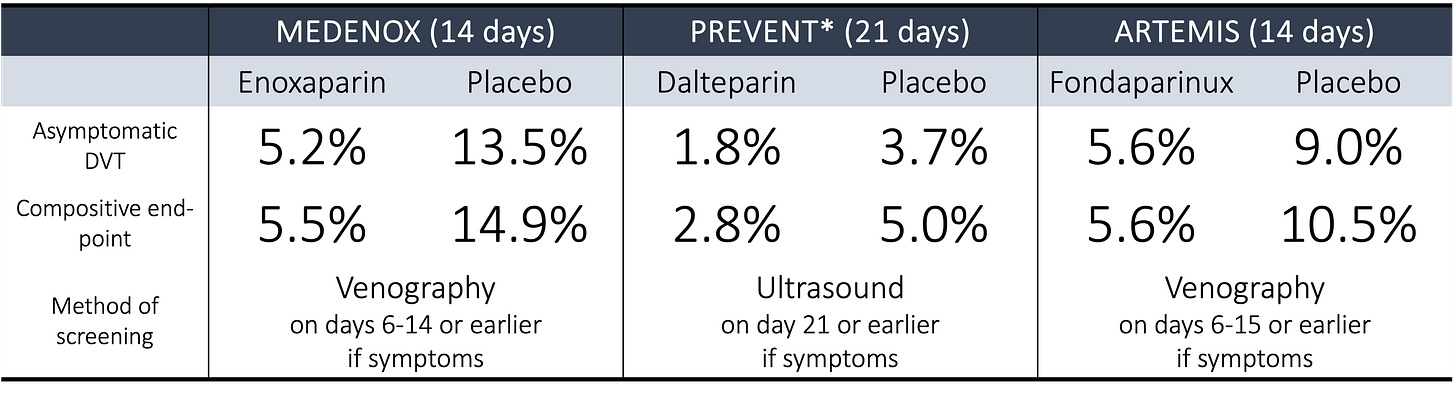
The modern evidence base was built on three pivotal, placebo-controlled trials conducted between 1999 and 2006. The first, MEDENOX, randomized 1102 hospitalized patients felt at moderate risk1 for VTE to 40 mg of enoxaparin, 20 mg of enoxaparin, or placebo once daily for 6 to 14 days. Published in The New England Journal of Medicine, MEDENOX reported the following results (these are the results for the 40 mg enoxaparin arm, as the 20 mg arm showed no benefit):
None of the outcomes were statistically significant.
A Consensus Emerges
Armed with this evidence, the Sixth ACCP Consensus Conference on Antithrombotic Therapy (published in Chest in 2001) issued a Grade 1A directive to give low-dose unfractionated heparin (LDUH) or low-molecular-weight heparin (LMWH) to at-risk medical patients, formally placing them on equal footing with surgical populations. The Seventh ACCP Guidelines, published in 2004, reaffirmed and broadened that stance, adding fondaparinux as an alternative. Soon after, the Joint Commission and CMS created the VTE Core Measure Set, making timely prophylaxis or documented contraindication a reportable quality metric for U.S. hospitals and, ultimately, a condition of accreditation.
The results of MEDENOX were followed by PREVENT (published in 2004 in Circulation and using dalteparin) and ARTEMIS (published in 2006 in The British Medical Journal and using fondaparinux).
Here are the results from PREVENT:
And ARTEMIS:
None of the outcomes shown above for PREVENT were statistically significant. ARTEMIS did show a reduction in fatal PE at 15 days (0% versus 1.2%, p = 0.029), though the methods by which the diagnosis of a fatal PE was made are suspect.2
What’s Missing?
When I first reviewed these studies in detail, I was surprised. How could these trials, where just one showed a reduction in symptomatic VTE, lead to a change in the standard of care, IA recommendations from multiple societies, and a Joint Commission metric for accreditation? The explanation comes down to a surrogate end-point: asymptomatic DVT.
All three trials screened patients for asymptomatic DVT at 14 or 21 days, either using venography or ultrasound. Even if patients reported no new symptoms, they were assessed to look for silent disease. To those readers who do not practice inpatient medicine, screening for DVT in the hospital has never been the standard of care, has never been recommended in guidelines, and has no evidence base supporting it. And yet, this outcome was included. And here’s what the three landmark trials showed:
When looking at these numbers, it becomes clear that asymptomatic DVT drove the primary outcome in all three trials. These were, far and away, the most common events documented.
The question then becomes, why was asymptomatic DVT included in these trials? The Ninth ACCP guidelines offer two rationales: “The first is that most thrombi start as small calf vein thrombi, which often remain asymptomatic but can grow to form symptomatic venous thrombi, which in turn can break off to cause asymptomatic PE, symptomatic PE, or fatal PE. The second is that a reduction in asymptomatic venous thrombosis by antithrombotic prophylaxis is paralleled by a similar reduction in symptomatic VTE and fatal PE.”
A more pointed explanation is offered by Sonaglia et al, who observe that “In studies of the prevention of postoperative DVT, the use of a clinical end point is problematic because of the low incidence of these events (and the large dimension of the required sample size).” And MEDENOX, PREVENT, and ARTEMIS demonstrate well that asymptomatic DVT is common in sick hospitalized patients. Depending on the modality used, between 4% and 14% of patients in the placebo arms were found to have asymptomatic DVT.
A number of questions emerge:
What is the clinical significance of an asymptomatic DVT (i.e., how likely is it to become a symptomatic DVT, embolize and cause a pulmonary embolism, or cause death)?
Does treating asymptomatic DVT improve outcomes?
Next, I’ll examine these two questions.
What is the clinical significance of an asymptomatic DVT?
As one may imagine, it is hard to study the natural history of an asymptomatic DVT. Once identified, proximal DVTs are likely to be treated, and even some distal DVTs will prompt the initiation of therapeutic anticoagulation. As a result, there are a paucity of cohorts demonstrating the evolution of this condition.
One of the most widely cited studies was published in 1969 in The Lancet. It included 132 consecutive postoperative patients, all of whom underwent screening for DVT. Among the 40 patients found to have a DVT, 14 (35%) lysed spontaneously within seventy-two hours. Although the remaining 26 (65%) of patients were found to have persistent thrombosis, most (74%) remained localized to the calf and did not extend proximally. Pulmonary embolism was diagnosed in 4 (10%) patients. These older data suggest that even without treatment, (a) not all DVTs persist or progress; (b) distal disease is particularly unlikely to progress; (c) pulmonary embolism is relatively rare.
Subsequent studies support the premise that asymptomatic distal DVTs are very unlikely to embolize. For example, a 1981 study found that none of the 21 patients with distal DVT developed pulmonary embolism. The best data comes from surgical studies, where approximately 10% to 20% of distal DVTs have been shown to extend to the proximal veins or cause pulmonary embolism.
Another way to estimate the risk of the proportion of asymptomatic DVTs that become symptomatic is to look at the ratio between symptomatic and asymptomatic disease. A 2015 study by Chan et al. reported a ratio of 10:1 for general medical patients. They write that “the practical implication of our findings is that asymptomatic DVT is not a reliable surrogate for symptomatic events.” Yet, the key data supporting VTE chemoprophylaxis rests on this endpoint.
Does treating asymptomatic DVT improve outcomes?
As with the prior question, answers to this one are elusive. Unlike apparently asymptomatic spinal stenosis or infiltrates on chest imaging, we don’t typically obtain ultrasounds of the leg veins unless a patient has symptoms suggesting DVT. This means that we’re left, again, with studies where patients were screened. Even here, the data is sparse. A 1997 study of orthopedic patients who were receiving postoperative warfarin prophylaxis utilized screening ultrasonography with subsequent treatment of identified asymptomatic DVT. The authors reported no difference in the rate of subsequent symptomatic VTE.
The Ninth ACCP guidelines realize this, writing that “In a population with a low prevalence of DVT, such as medical patients, even with a highly-specific test such as ultrasound, one would anticipate a substantial number of false-positive results. Moreover, even without considering false-positive results, routine ultrasound screening would be associated with appreciable cost and inconvenience without evidence of benefit.”
What do more recent studies show?
In the years after MEDENOX, uncertainties were raised about the effect of chemoprophylaxis on mortality. To address these questions, patients were randomized, once again, to 40 mg of enoxaparin daily or placebo, both administered for 10±4 days. The results of the LIFENOX trial were published in 2011 in The New England Journal of Medicine. The authors reported that the rate of death from any cause at day 30 was 4.9% in the enoxaparin group and 4.8% in the placebo group.
What I find amazing is that the authors did not think it important to formally report rates of symptomatic DVT or PE. All you are offered is that “Up to day 90, there was clinical suspicion of venous thromboembolism in 0.5% of the patients in the enoxaparin group (22 of 4072 patients) and in 0.7% of the patients in the placebo group (27 of 4044 patients). The diagnosis was confirmed by objective testing in 0.2% of the patients in the enoxaparin group and in 0.1% of the patients in the placebo group.” So, if I read this correctly, LIFENOX did not demonstrate a difference in the rate of symptomatic VTE. Why isn’t this notable?
More recently, the SYMPTOMS trial was published in 2023 in NEJM Evidence. In this trial, older adults (>70 years of age) hospitalized for acute medical conditions were randomly assigned to either 40 mg of enoxaparin daily or placebo for 6 to 14 days. The primary efficacy outcome was the cumulative incidence of symptomatic VTE (distal or proximal deep vein thrombosis, fatal or nonfatal pulmonary embolism) at 30 days. Here are the results:
At 30 days, there was no difference in rates of the primary outcomes or in each of its composites (distal DVT, proximal DVT, PE, or fatal PE). At 90 days, there is a suggestion of a benefit of enoxaparin, but this did not reach statistical significance. Once again, there is certainly no difference in mortality at 90 days.
Conclusion
As I have argued elsewhere, I worry that the provision of VTE chemoprophylaxis is a Thing We Do For No Reason. Although the use of asymptomatic DVT as a surrogate endpoint in initial studies seems justified, to then use these data as the basis for guideline recommendations and Joint Commission standards seems, to me, premature. Instead, it would seem more prudent to take the results of studies like MEDENOX and use them to justify larger studies where the primary efficacy endpoint is symptomatic VTE. Instead, the follow-up to MEDENOX (LIFENOX) utilized a reach endpoint (mortality) without first confirming a benefit in reducing symptomatic DVT or PE. And SYMPTOMS barely registered a murmur of interest, even though it appears to be the largest randomized trial of the most widely used chemoprophylaxis agent (enoxaparin) to focus on the key outcomes of interest.
I am not optimistic that we’ll see a change in the recommendations regarding VTE chemoprophylaxis for hospitalized medical patients. It remains the standard of care, and I utilize it in those deemed high-risk. Yet, I do this without strong confidence in the data that undergirds this practice.
Inclusion criteria included age >40 years, projected length of stay at least six days, and lack of immobilization for more than three days. To be eligible, patients had to have NYHA class III or IV heart failure, acute respiratory failure that did not require ventilatory support, or one of the following medical conditions if it was associated with at least one additional risk factor for venous thromboembolism: acute infection without septic shock; acute rheumatic disorders, including acute lumbar pain or sciatica or vertebral compression, acute arthritis of the legs, or an acute episode of rheumatoid arthritis in the legs; or an episode of inflammatory bowel disease. Exclusion criteria included stroke or major surgery within the previous three months, among others. These exclusions were made so that the highest-risk patients would not be randomized to placebo.
The methods note that “In the absence of autopsy, death was classified due to pulmonary embolism if it occurred suddenly and was otherwise unexplained.” Only two of the five fatal PEs were confirmed by autopsy. The other three were assumed to be due to pulmonary emboli, as no other plausible cause was found.